Copper matte consists of Cu2S along with some unchanged FeS. When a blast of hot air is passed through molten matte placed in silica lined converter, FeS present in matte is oxidised to FeO which combines with silica (SiO2) to form FeSiO3slag. 2S undergoes oxidation to form Cu20 which then reacts with more Cu2S to form copper metal. Thus, copper matte is heated in silica lined converter to remove FeS present in matte as FeSiO3 slag.
Copper matte consists of Cu2S along with some unchanged FeS. When a blast of hot air is passed through molten matte placed in silica lined converter, FeS present in matte is oxidised to FeO which combines with silica (SiO2) to form FeSiO3slag.
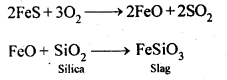 2S undergoes oxidation to form Cu20 which then reacts with more Cu2S to form copper metal.
2S undergoes oxidation to form Cu20 which then reacts with more Cu2S to form copper metal.
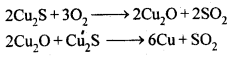
need an explanation for this answer? contact us directly to get an explanation for this answerThus, copper matte is heated in silica lined converter to remove FeS present in matte as FeSiO3 slag.